
Chemistry, 02.08.2019 22:30 rubimachuca1020
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.35 m, and the concentration of h2so3 is 0.23 m. this reaction a. is in equilibrium b. must shift to the reactants to be in equilibrium c. must shift to the products to be in equilibrium d. must have the pressure increased to reach equilibrium e. none of the above

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.3...
Questions




Mathematics, 23.05.2020 18:01





Biology, 23.05.2020 18:01

Mathematics, 23.05.2020 18:01

Mathematics, 23.05.2020 18:01

Mathematics, 23.05.2020 18:01

Mathematics, 23.05.2020 18:01

Mathematics, 23.05.2020 18:01






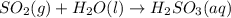
![Q=\frac{[H_2SO_3(aq)]}{[SO_2(g)]}](/tpl/images/0163/3606/a76c3.png)
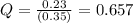
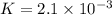
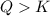 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.
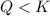 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.
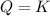 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

