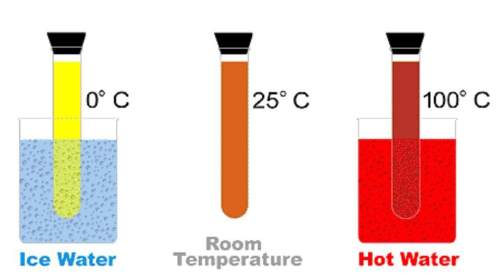
Using this reversible reaction, answer the following:
n2o4 < --> 2no2
(colorles...

Chemistry, 03.11.2019 20:31 cragajjar2
Using this reversible reaction, answer the following:
n2o4 < --> 2no2
(colorless) (reddish-brown)
-as the temperature increased, what happened to the [n2o4]?
-was the formation of reactants or products favored by the addition of heat?
-which reaction is exothermic?
-if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct?
n2o4 < --> 2no2 + 14 kcal
n2o4 < --> 2no2, hr = +14 kcal
n2o4 + 14 kcal < --> 2no2
n2o4 < --> 2no2, hr = -14 kcal

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1

Chemistry, 23.06.2019 13:30
If a fast moving car making a loud noise approaches and moves past the person what will happen as the distance between the two increases?
Answers: 1
You know the right answer?
Questions


Engineering, 02.10.2019 17:20


Health, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30


History, 02.10.2019 17:30


Computers and Technology, 02.10.2019 17:30


English, 02.10.2019 17:30



Mathematics, 02.10.2019 17:30



Engineering, 02.10.2019 17:30

Biology, 02.10.2019 17:30




