Given the reaction system in a closed container at equilibrium and at a temperature of 298 k:
...

Chemistry, 13.01.2020 23:31 smelcher3900
Given the reaction system in a closed container at equilibrium and at a temperature of 298 k:
n2o4(g) < ==> 2no2(g)
the measurable quantities of the gases at equilibrium must be
(1) decreasing (3) equal
(2) increasing (4) constant

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
Questions

Mathematics, 09.09.2021 19:00

Physics, 09.09.2021 19:00


Chemistry, 09.09.2021 19:00

Mathematics, 09.09.2021 19:00



Mathematics, 09.09.2021 19:00

Mathematics, 09.09.2021 19:00

Chemistry, 09.09.2021 19:00

Advanced Placement (AP), 09.09.2021 19:00


Mathematics, 09.09.2021 19:00

Biology, 09.09.2021 19:00

English, 09.09.2021 19:00


Business, 09.09.2021 19:00


Social Studies, 09.09.2021 19:00

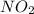 , rate of formation of
, rate of formation of 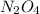 , pressure of
, pressure of 

