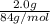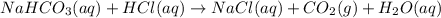If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon dioxide should one tablet yield? compare this theoretical value with your results. (hint: you will first need to convert your mass into moles by dividing by the molar mass of nahco3 or sodium hydrogen carbonate a/k/a baking soda). , show all work and think about mole ratios relating co2 to nahco3.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon di...
Questions


Social Studies, 25.05.2020 10:58

Mathematics, 25.05.2020 10:58


Mathematics, 25.05.2020 10:58



Mathematics, 25.05.2020 10:58




Physics, 25.05.2020 10:58


Mathematics, 25.05.2020 10:58


Mathematics, 25.05.2020 10:58


Mathematics, 25.05.2020 10:58

Mathematics, 25.05.2020 10:58

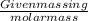 (1)
(1)