Chemistry, 02.08.2019 14:00 jslaughter3
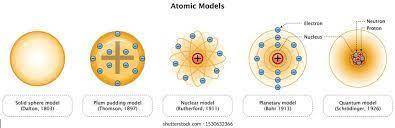
Thomson's atomic model is best described by which of the following statements? (2 points) a nucleus with electrons moving around it like planets a positive solid sphere with electrons dispersed throughout neutrons surrounded by orbiting electrons a negative sphere with electrons surrounding it 2. which of the following is not part of dalton's atomic theory? (2 points) all matter is made up of atoms. all atoms of a given element are the same. different elements are made up of different atoms. atoms can be broken down into smaller pieces. 3. what describes the current model of the atom? (2 points) protons orbit the nucleus like planets orbit the sun. that electrons and protons move randomly around a nucleus. electrons travel as waves in the electron cloud that surrounds the nucleus. electrons orbit the nucleus like planets orbit the sun. 4. consider the work of thomson, rutherford, and bohr. in your opinion, which was the most important and why? be sure to state what your selected person discovered and how it contributed to the model of the atom. (4 points)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
Thomson's atomic model is best described by which of the following statements? (2 points) a nucleus...
Questions


Mathematics, 01.04.2021 19:00








History, 01.04.2021 19:00



Mathematics, 01.04.2021 19:00




Chemistry, 01.04.2021 19:00

Mathematics, 01.04.2021 19:00