
Chemistry, 02.08.2019 09:30 sammamamam7905
The atomic masses of 79 br 35 (50.69 percent) and 81 br 35 (49.31 percent) are 78.9183361 amu and 80.916289 amu, respectively. calculate the average atomic mass of bromine. the percentages in parentheses denote the relative abundances.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
The atomic masses of 79 br 35 (50.69 percent) and 81 br 35 (49.31 percent) are 78.9183361 amu and 80...
Questions

History, 07.04.2021 03:20

Mathematics, 07.04.2021 03:20

Mathematics, 07.04.2021 03:20



Biology, 07.04.2021 03:20


Mathematics, 07.04.2021 03:20

Mathematics, 07.04.2021 03:20

History, 07.04.2021 03:20



Advanced Placement (AP), 07.04.2021 03:20


Mathematics, 07.04.2021 03:20





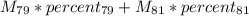 . This yields an average atomic mass of 79.903 amu. This procedure is general; whenever we have many types F of a specific genre G, the mean of the genre G can be calculated by:
. This yields an average atomic mass of 79.903 amu. This procedure is general; whenever we have many types F of a specific genre G, the mean of the genre G can be calculated by: 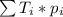
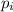 is the proportion of type i in the genre G. In this example, Br79 and Br81 are the 2 types and the genre is Bromine.
is the proportion of type i in the genre G. In this example, Br79 and Br81 are the 2 types and the genre is Bromine.

