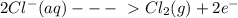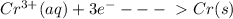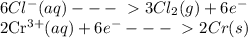Chemistry, 02.08.2019 05:30 orlando19882000
The information below describes a redox reaction. cr3+(aq)+2cl-(> cr(s)+cl2(s) 2cl-(> cl2(g)+2e- cr3+(aq)+3e- > cr(s) what is the final, balanced equation for this reaction? 1.) 2cr3+(aq)+6cl-(aq) > 2cr(s)+3cl2(g) 2.) 2cr3(aq)+2cl-(aq)+6e- > cl2(g)+2cr(s) 3.) cr3+(aq)+6cl-(aq)+3e- > 2cr(g)+3cl2(g) 4.) cr3+(aq)+2cl-(> cr(s)+cl2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


You know the right answer?
The information below describes a redox reaction. cr3+(aq)+2cl-(> cr(s)+cl2(s) 2cl-(> cl2(g)+2...
Questions

Mathematics, 01.08.2019 04:50

Mathematics, 01.08.2019 04:50


Mathematics, 01.08.2019 04:50


Mathematics, 01.08.2019 04:50

Business, 01.08.2019 04:50


Biology, 01.08.2019 04:50

History, 01.08.2019 04:50

Business, 01.08.2019 04:50

History, 01.08.2019 04:50

Physics, 01.08.2019 04:50

Mathematics, 01.08.2019 04:50

Chemistry, 01.08.2019 04:50

Mathematics, 01.08.2019 04:50

Chemistry, 01.08.2019 04:50


Mathematics, 01.08.2019 04:50

Chemistry, 01.08.2019 04:50