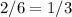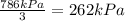Chemistry, 02.08.2019 04:30 gabbym39077
When a container is filled with 3 moles of helium gas, 2 moles of oxygen gas, and 1 mole of carbon dioxide gas, the pressure in the container is 786 kpa. what is the partial pressure of the oxygen gas? a. 131 kpa b. 262 kpa c. 393 kpa d. 786 kpa

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
When a container is filled with 3 moles of helium gas, 2 moles of oxygen gas, and 1 mole of carbon d...
Questions

History, 09.03.2021 20:50

Mathematics, 09.03.2021 20:50


Mathematics, 09.03.2021 20:50




Physics, 09.03.2021 20:50

Mathematics, 09.03.2021 20:50




Mathematics, 09.03.2021 20:50

Mathematics, 09.03.2021 20:50

Mathematics, 09.03.2021 20:50


Geography, 09.03.2021 20:50

Mathematics, 09.03.2021 20:50