
Chemistry, 01.08.2019 23:00 asilverman
The half-life of cobalt-60 is 5.20 yr. how many milligrams of a 1.000-mg sample remain after 6.55 years?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Asap! ! who wants to me with my assignment : ) (especially with how i’m gonna draw out the example) you have explored some interesting, informative, and amusing examples of models. now it's time to get creative and make your own model. here is the requirement checklist for your model: ✔ model types can include drawings, diagrams, physical models, virtual simulations, or videos. ✔ models must be created by you, not something selected from an online or outside source. ✔ submit a presentation, picture, video, or screenshot of your model. ✔ submit a one-paragraph summary describing the topic you chose, your model, what it represents, how you made it, and the specific science involved. it is important that you are using science terminology and are accurate. now that you know how to create and submit your model, you will need to choose a topic for your model. choose one of the three topics listed below. select each topic for an overview. -conservation of mass -atomic theory -thermal energy
Answers: 2

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2
You know the right answer?
The half-life of cobalt-60 is 5.20 yr. how many milligrams of a 1.000-mg sample remain after 6.55 ye...
Questions






History, 04.04.2020 13:12


Advanced Placement (AP), 04.04.2020 13:13


Mathematics, 04.04.2020 13:13



Computers and Technology, 04.04.2020 13:14







Computers and Technology, 04.04.2020 13:14

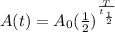 . Here,
. Here, 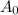 is the initial quantity,
is the initial quantity, 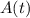 is the amount remaining after a time
is the amount remaining after a time 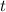 , and
, and 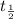 is the half-life of the decaying quantity. You can plug in 1.000 for
is the half-life of the decaying quantity. You can plug in 1.000 for 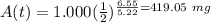 . Be mindful of significant figures if your instructor cares about that.
. Be mindful of significant figures if your instructor cares about that.

