

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

You know the right answer?
In the following combustion reaction, what is the mole ratio of diatomic oxygen to water? 2 c2h6 + 7...
Questions


Mathematics, 17.12.2020 18:10


Mathematics, 17.12.2020 18:10


Mathematics, 17.12.2020 18:10

Mathematics, 17.12.2020 18:10



Mathematics, 17.12.2020 18:10









Mathematics, 17.12.2020 18:10

Health, 17.12.2020 18:10

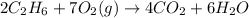
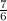 .
.

