
Chemistry, 01.08.2019 11:00 deannajd03
A0.477 mol sample of o2 gas has a volume of 12.1 l at a certain temperature and pressure. if all this o2 were converted to ozone (o3 ) at the same temperature and pressure, what is the ozone volume (in liters)? 3o2 (g) → 2o3 (g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2
You know the right answer?
A0.477 mol sample of o2 gas has a volume of 12.1 l at a certain temperature and pressure. if all th...
Questions


History, 31.01.2020 15:42


Mathematics, 31.01.2020 15:42


Mathematics, 31.01.2020 15:42

Mathematics, 31.01.2020 15:42




Mathematics, 31.01.2020 15:42


Biology, 31.01.2020 15:42

Mathematics, 31.01.2020 15:42

History, 31.01.2020 15:42

Mathematics, 31.01.2020 15:42

Mathematics, 31.01.2020 15:42

History, 31.01.2020 15:42


Mathematics, 31.01.2020 15:42

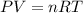 , is very helpful. The number of moles
, is very helpful. The number of moles 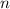 changes here by a factor of
changes here by a factor of 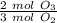 , so you have
, so you have 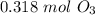 .
. 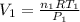 and
and 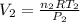 . Looking at the given information, P and T are constant (as is R, always). So, we can write
. Looking at the given information, P and T are constant (as is R, always). So, we can write 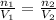 . Solving for
. Solving for 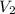 , we get
, we get 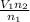 . Then we plug in 12.1 L for
. Then we plug in 12.1 L for 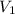 ,
, 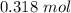 for
for 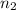 and
and 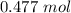 for
for 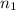 . Thus
. Thus 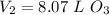 .
.

