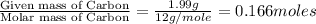Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
An unknown compound contains only carbon, hydrogen, and oxygen (cxhyoz). combustion of 5.00 g of thi...
Questions






History, 05.05.2020 13:00

Mathematics, 05.05.2020 13:00

Mathematics, 05.05.2020 13:00



Physics, 05.05.2020 13:00

Mathematics, 05.05.2020 13:00

Mathematics, 05.05.2020 13:00

Mathematics, 05.05.2020 13:00

Mathematics, 05.05.2020 13:00




Biology, 05.05.2020 13:00

English, 05.05.2020 13:00

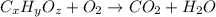
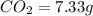
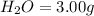
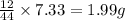 of carbon will be contained.
of carbon will be contained.