
Chemistry, 01.08.2019 04:30 natalie407888
Chemistry ! : neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic mass of 40.0 g/mol. how would the number of atoms in a 1.0 mol sample of neon compare to the number of atoms in a 4.0 mol sample of argon? (2 points) a. the argon sample would have the same number of atoms as the neon sample. b. the argon sample would have twice as many atoms as the neon sample. c. the argon sample would have four times as many atoms as the neon sample. d. the argon sample would have eight times as many atoms as the neon sample.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Chemistry ! : neon has an average atomic mass of 20.2 g/mol, whereas argon has an average atomic m...
Questions

Mathematics, 22.12.2019 20:31






History, 22.12.2019 20:31

Mathematics, 22.12.2019 20:31

Mathematics, 22.12.2019 20:31


Mathematics, 22.12.2019 20:31









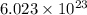 of particles.
of particles.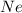 contains=
contains=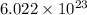 atoms
atoms 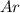 contains=
contains=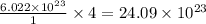 atoms
atoms

