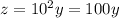Chemistry, 17.10.2019 10:10 BrodsterBj
The ph of a solution decreases by 2.0. how does the hydronium ion concentration of the solution change?
increases to 2 times the original concentration
increases to 100 times the original concentration
decreases to 1/100 of the original concentration
decreases to 1/2 of the original concentration

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
The ph of a solution decreases by 2.0. how does the hydronium ion concentration of the solution chan...
Questions



Spanish, 25.05.2021 01:00


English, 25.05.2021 01:00

Chemistry, 25.05.2021 01:00





Mathematics, 25.05.2021 01:00








Mathematics, 25.05.2021 01:00

![[H^+]](/tpl/images/0328/0396/07acb.png) initially present be y
initially present be y![x=-log[y]](/tpl/images/0328/0396/acf48.png) ...(1)
...(1)![x-2=-log[z]](/tpl/images/0328/0396/38f29.png) ...(2)
...(2)![-log[y]-2=-log[z]](/tpl/images/0328/0396/aa742.png)