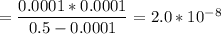Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Calculate the acid dissociation constant of a weak monoprotic acid if a 0.5m solution of this acid g...
Questions



Mathematics, 18.03.2021 03:40

Mathematics, 18.03.2021 03:40

Mathematics, 18.03.2021 03:40




English, 18.03.2021 03:40

Business, 18.03.2021 03:40

Spanish, 18.03.2021 03:40

Mathematics, 18.03.2021 03:40

Mathematics, 18.03.2021 03:40

Mathematics, 18.03.2021 03:40



Mathematics, 18.03.2021 03:40


Mathematics, 18.03.2021 03:40

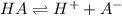
![K_a= \dfrac{[H^+][A^-]}{[HA]}](/tpl/images/0155/5739/a4583.png)
![0.5M=[A^-]+[HA]](/tpl/images/0155/5739/14b10.png)
![[HA]=0.5M-[A^-]](/tpl/images/0155/5739/ce8c0.png)
![K_a= \dfrac{[H^+][A^-]}{0.5M-[A^-]}](/tpl/images/0155/5739/a476b.png)