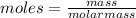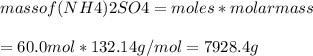Chemistry, 31.07.2019 20:00 chamyaparker
The chemical equation shows how ammonia reacts with sulfuric acid to produce ammonium sulfate. 2nh3(aq) + h2so4(aq) (nh4)2so4(aq) how many grams of ammonium sulfate can be produced if 60.0 mol of sulfuric acid react with an excess of ammonia? 1,020 g 3,970 g 5,890 g 7,930 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
The chemical equation shows how ammonia reacts with sulfuric acid to produce ammonium sulfate. 2nh3(...
Questions

Advanced Placement (AP), 28.01.2021 18:40


Geography, 28.01.2021 18:40



History, 28.01.2021 18:40

Mathematics, 28.01.2021 18:40


History, 28.01.2021 18:40



Biology, 28.01.2021 18:40

Mathematics, 28.01.2021 18:40

Mathematics, 28.01.2021 18:40

Social Studies, 28.01.2021 18:40



Mathematics, 28.01.2021 18:40

Mathematics, 28.01.2021 18:40

Biology, 28.01.2021 18:40