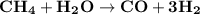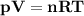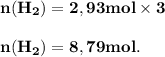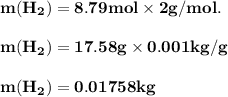Chemistry, 31.07.2019 18:30 katlynnschmolke
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. suppose a chemical engineer studying a new catalyst for the reform reaction finds that 159. liters per second of methane are consumed when the reaction is run at 294.°c and 0.86atm . calculate the rate at which dihydrogen is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3
You know the right answer?
The reform reaction between steam and gaseous methane ( ch4 ) produces "synthesis gas," a mixture of...
Questions

Biology, 30.01.2020 13:59






Mathematics, 30.01.2020 13:59

Health, 30.01.2020 13:59


Mathematics, 30.01.2020 13:59

Social Studies, 30.01.2020 13:59

Biology, 30.01.2020 13:59




Arts, 30.01.2020 13:59

World Languages, 30.01.2020 13:59



Mathematics, 30.01.2020 13:59