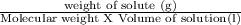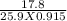Chemistry, 31.07.2019 14:00 keatonjarvis
What would be the resulting molaritybof a solution made by dissolving 17.8 g of lif in enough water to make a 915-millimeter solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2


Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
What would be the resulting molaritybof a solution made by dissolving 17.8 g of lif in enough water...
Questions

Mathematics, 29.01.2021 18:20

Advanced Placement (AP), 29.01.2021 18:20

Mathematics, 29.01.2021 18:20

History, 29.01.2021 18:20






Mathematics, 29.01.2021 18:20

English, 29.01.2021 18:20


Mathematics, 29.01.2021 18:20


Mathematics, 29.01.2021 18:20

Chemistry, 29.01.2021 18:20




English, 29.01.2021 18:20