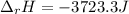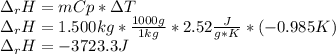Chemistry, 31.07.2019 12:00 anabelleacunamu
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbon dioxide (co2), and water (h2o). a chemist carries out this reaction in a bomb calorimeter. the reaction causes the temperature of a bomb calorimeter to decrease by 0.985 k. the calorimeter has a mass of 1.500 kg and a specific heat of 2.52 j/g•k. what is the heat of reaction for this system?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

You know the right answer?
Sodium carbonate (na2co3) reacts with acetic acid (ch3cooh) to form sodium acetate (nach3coo), carbo...
Questions

Mathematics, 12.10.2019 12:20

Mathematics, 12.10.2019 12:20

Mathematics, 12.10.2019 12:20





History, 12.10.2019 12:20

History, 12.10.2019 12:20




English, 12.10.2019 12:20







Mathematics, 12.10.2019 12:20