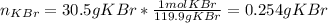Chemistry, 31.07.2019 11:00 QuestionAsker4356
What volume of a 0.716 m kbr solution is needed to provide 30.5 g of kbr?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
What volume of a 0.716 m kbr solution is needed to provide 30.5 g of kbr?...
Questions


English, 17.02.2022 20:00

History, 17.02.2022 20:00



Social Studies, 17.02.2022 20:00

SAT, 17.02.2022 20:00





Mathematics, 17.02.2022 20:00

Chemistry, 17.02.2022 20:10



English, 17.02.2022 20:10


Mathematics, 17.02.2022 20:10

History, 17.02.2022 20:10

Spanish, 17.02.2022 20:10