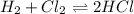Chemistry, 31.07.2019 07:00 nayelimoormann
Consider the reaction h2(g) + cl2(g) ⇀↽ 2 hcl(g), which is exothermic as written. what would be the effect on the equilibrium position of increasing the temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Consider the reaction h2(g) + cl2(g) ⇀↽ 2 hcl(g), which is exothermic as written. what would be the...
Questions



Mathematics, 31.07.2020 01:01



Mathematics, 31.07.2020 01:01

Mathematics, 31.07.2020 01:01



Mathematics, 31.07.2020 01:01


Mathematics, 31.07.2020 01:01






Mathematics, 31.07.2020 01:01

History, 31.07.2020 01:01

Mathematics, 31.07.2020 01:01