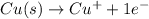Chemistry, 31.07.2019 06:30 Manuelperez1373
In the balanced redox reaction: 2 cu(s) + s(s) ® cu2s(s), how many electrons are gained or lost by each copper atom? select one: a. each copper atom gains two (2) electrons. b. each copper atom gains one (1) electron. c. each copper atom loses one (1) electron. d. each copper atom loses two (2) electrons.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
In the balanced redox reaction: 2 cu(s) + s(s) ® cu2s(s), how many electrons are gained or lost by...
Questions

Mathematics, 28.07.2019 14:00


Health, 28.07.2019 14:00




History, 28.07.2019 14:00

Social Studies, 28.07.2019 14:00

Geography, 28.07.2019 14:00

Mathematics, 28.07.2019 14:00

Physics, 28.07.2019 14:00


Mathematics, 28.07.2019 14:00

Biology, 28.07.2019 14:00



Mathematics, 28.07.2019 14:00



Physics, 28.07.2019 14:00