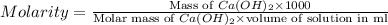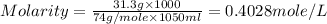Chemistry, 31.07.2019 05:00 mzlennon639
Question 8 4 pts what would be the resulting molarity of a solution made by dissolving 31.3 grams of ca(oh)2 in enough water to make a 1050-milliliter solution? show all of the work needed to solve this problem.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
Question 8 4 pts what would be the resulting molarity of a solution made by dissolving 31.3 grams of...
Questions

History, 09.10.2019 18:20





Mathematics, 09.10.2019 18:20




Chemistry, 09.10.2019 18:20





English, 09.10.2019 18:30





Mathematics, 09.10.2019 18:30

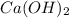 = 31.3 g
= 31.3 g