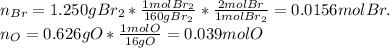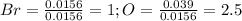Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 10:30
What is the difference between skimming and absorbing methods of the oil removal
Answers: 2

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
Oxides of virtually every element are known. bromine, for example, forms several oxides when treated...
Questions

Mathematics, 23.04.2020 20:10




History, 23.04.2020 20:10

English, 23.04.2020 20:10

Mathematics, 23.04.2020 20:10


Mathematics, 23.04.2020 20:10


Mathematics, 23.04.2020 20:10

Mathematics, 23.04.2020 20:10



Mathematics, 23.04.2020 20:11

Mathematics, 23.04.2020 20:11


Health, 23.04.2020 20:11

Mathematics, 23.04.2020 20:11

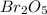

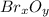 because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the
because the grams of bromine are equal before and after the chemical reaction (mass can't be neither created nor destroyed), thus, the bromine grams into the