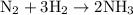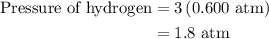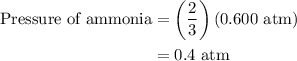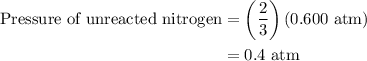Chemistry, 30.07.2019 22:00 tiffanibell71
Arigid vessel at constant temperature initially contains 0.600 atm nitrogen gas and 0.600 atm hydrogen gas. if these gases react to form ammonia and the reaction goes to completion, which choice is closest to the final total pressure after the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Arigid vessel at constant temperature initially contains 0.600 atm nitrogen gas and 0.600 atm hydrog...
Questions

Mathematics, 21.01.2021 19:10



Mathematics, 21.01.2021 19:10



Mathematics, 21.01.2021 19:10

History, 21.01.2021 19:10

Biology, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Health, 21.01.2021 19:10



Mathematics, 21.01.2021 19:10

History, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

English, 21.01.2021 19:10


Mathematics, 21.01.2021 19:10

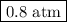 .
.