

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Asolution of hcl in water conducts electricity, but a solution of hcl in chloroform, chcl3, does not...
Questions


Mathematics, 10.10.2019 02:00





Chemistry, 10.10.2019 02:00

Biology, 10.10.2019 02:00


Arts, 10.10.2019 02:00

History, 10.10.2019 02:00


Law, 10.10.2019 02:00


Biology, 10.10.2019 02:00

Mathematics, 10.10.2019 02:00


Mathematics, 10.10.2019 02:00


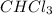 is a non-polar compound and when HCl is added to it then HCl being polar in nature is unable to dissolve.
is a non-polar compound and when HCl is added to it then HCl being polar in nature is unable to dissolve.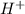 and
and 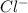 ions. And, HCl in
ions. And, HCl in 

