
Chemistry, 30.07.2019 13:00 chantelljenkins2
Assume that 1.0 mol of c4h10 is completely burned in excess oxygen to form carbon dioxide and water. how many moles of co2 would be produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1
You know the right answer?
Assume that 1.0 mol of c4h10 is completely burned in excess oxygen to form carbon dioxide and water....
Questions

Mathematics, 25.10.2021 07:50

Mathematics, 25.10.2021 07:50


Mathematics, 25.10.2021 07:50


Mathematics, 25.10.2021 08:00

Mathematics, 25.10.2021 08:00

Biology, 25.10.2021 08:00



English, 25.10.2021 08:00

Mathematics, 25.10.2021 08:00





Mathematics, 25.10.2021 08:00



Biology, 25.10.2021 08:00

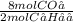 ) = 4 moles of CO₂
) = 4 moles of CO₂

