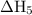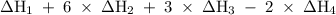Chemistry, 30.07.2019 13:00 emacwhaleng
Consider these reactions, where m represents a generic metal. 2m(s)+6hcl(aq)⟶2mcl3(aq)+3h2(g)δh1= −556.0 kj hcl(g)⟶hcl(aq) δh2=−74.8 kj h2(g)+cl2(g)⟶2hcl(g) δh3=−1845.0 kj mcl3(s)⟶mcl3(aq) δh4=−342.0 kj use the given information to determine the enthalpy of the reaction 2m(s)+3cl2(g)⟶2mcl3(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Consider these reactions, where m represents a generic metal. 2m(s)+6hcl(aq)⟶2mcl3(aq)+3h2(g)δh1= −5...
Questions



Spanish, 17.10.2020 01:01

Mathematics, 17.10.2020 01:01




History, 17.10.2020 01:01

Mathematics, 17.10.2020 01:01

Mathematics, 17.10.2020 01:01

Mathematics, 17.10.2020 01:01


Mathematics, 17.10.2020 01:01

Mathematics, 17.10.2020 01:01

History, 17.10.2020 01:01


Mathematics, 17.10.2020 01:01


Mathematics, 17.10.2020 01:01

Chemistry, 17.10.2020 01:01

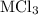 will be -5849 kJ.
will be -5849 kJ.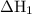 = -556 kJ
= -556 kJ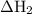 = -74.8 kJ
= -74.8 kJ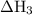 = -1845 kJ
= -1845 kJ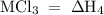 = -342.0 kJ
= -342.0 kJ