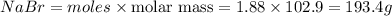Chemistry, 16.12.2019 02:31 devbar3416
In the following reaction, how many grams of nabr will react with 311 grams of pb(no3)2?
pb(no3)2(aq)+2nabr(g) -> pbbr2(s) + 2nano3(aq)
the molar mass of nabr is 102.9 grams and that of pb(no3)2 is 331.21 grams.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
In the following reaction, how many grams of nabr will react with 311 grams of pb(no3)2?
pb(no...
pb(no...
Questions

History, 12.07.2019 13:30

Mathematics, 12.07.2019 13:30


History, 12.07.2019 13:30


History, 12.07.2019 13:30


Social Studies, 12.07.2019 13:30


History, 12.07.2019 13:30


Business, 12.07.2019 13:30



Biology, 12.07.2019 13:30





Biology, 12.07.2019 13:30

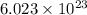 of particles.
of particles.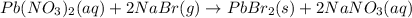
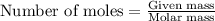
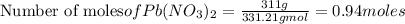
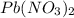 reacts with 2 moles of
reacts with 2 moles of 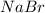
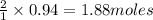 of
of