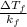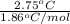Chemistry, 30.07.2019 02:00 vanessa051266
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 2.75 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 2.75 ∘c. what is the molal concentration...
Questions

English, 13.04.2021 01:00

Mathematics, 13.04.2021 01:00

Mathematics, 13.04.2021 01:00

Mathematics, 13.04.2021 01:00




Mathematics, 13.04.2021 01:00

English, 13.04.2021 01:00

Mathematics, 13.04.2021 01:00

Mathematics, 13.04.2021 01:00


Mathematics, 13.04.2021 01:00


Mathematics, 13.04.2021 01:00




Mathematics, 13.04.2021 01:00

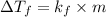
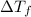 = difference in temperature =
= difference in temperature = ![[0 - (-2.75)]^{o}C](/tpl/images/0148/8342/d4fc3.png) =
=