
Chemistry, 30.07.2019 02:00 razielcornils04
This reaction was monitored as a function of time: ab --> a + b a plot of 1/[ab] versus time yields a straight line with slope of -0.055/m*s. a) what is the value of the rate constant (k) for this reaction t this temperature? b) write the rate law for the reaction. c) what is the half-life when the initial concentration is 0.55m? d) if the initial concentration of ab is 0.250m, and the reaction mixture initially contains no products, what are the concentrations of a and b after 75s?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
This reaction was monitored as a function of time: ab --> a + b a plot of 1/[ab] versus time yi...
Questions

History, 21.04.2020 22:46




Mathematics, 21.04.2020 22:46


Biology, 21.04.2020 22:46


Mathematics, 21.04.2020 22:46

World Languages, 21.04.2020 22:46

Mathematics, 21.04.2020 22:46









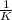 .
. 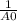
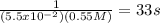
![\frac{1}{t} [ \frac{1}{[A]} - \frac{1}{[Ao]} ]](/tpl/images/0148/8063/927e3.png)


