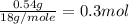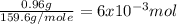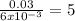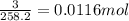Chemistry, 30.07.2019 02:00 maysahdabest
Suppose you begin with 1.50~g of the hydrate copper(ii)sulfate · x-hydrate (cuso4· x h2o), where x is an integer. after dehydration you find that you are left with 0.96~g of the an-hydrate cuso4. what is the unknown integer x. round your answer to the nearest integer, enter only an integer. suppose you begin with of the hydrate kal(so4)2 · 12h2o. after dehydration you find that you are left with 3.0~g of the an-hydrate kal(so4)2. how many grams did you start with? write the value to the correct number of significance figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Suppose you begin with 1.50~g of the hydrate copper(ii)sulfate · x-hydrate (cuso4· x h2o), where x i...
Questions


History, 27.08.2019 20:30


Mathematics, 27.08.2019 20:30

English, 27.08.2019 20:30


Health, 27.08.2019 20:30


Mathematics, 27.08.2019 20:30


Mathematics, 27.08.2019 20:30




Biology, 27.08.2019 20:30

Mathematics, 27.08.2019 20:30


Social Studies, 27.08.2019 20:30

Mathematics, 27.08.2019 20:30

Biology, 27.08.2019 20:30