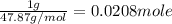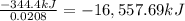The combustion of titanium with oxygen produces titanium dioxide: ti (s) + o2(g) â tio2 (s) when 1.000 g of titanium is combusted in a bomb calorimeter, the temperature of the calorimeter increases from 25.00 â°c to 60.00 â°c. in a separate experiment, the heat capacity of the calorimeter is measured to be 9.84 kj/k. the heat of reaction for the combustion of a mole of ti in this calorimeter is kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
The combustion of titanium with oxygen produces titanium dioxide: ti (s) + o2(g) â tio2 (s) when 1....
Questions

World Languages, 27.10.2021 02:40

Social Studies, 27.10.2021 02:40





Engineering, 27.10.2021 02:40

Physics, 27.10.2021 02:40


Mathematics, 27.10.2021 02:40

Mathematics, 27.10.2021 02:40


Biology, 27.10.2021 02:40



Biology, 27.10.2021 02:40

Geography, 27.10.2021 02:40

Mathematics, 27.10.2021 02:40

Mathematics, 27.10.2021 02:40