
Chemistry, 29.07.2019 16:30 julieariscar769
Assuming that the density of vinegar is 1.005 g/ml, calculate the molarity of acetic acid in vinegar from your average value for the mass percentage of acetic acid in vinegar. density of vinegar= 1.005 g/ml average mass % acetic acid= 5.2%,

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Assuming that the density of vinegar is 1.005 g/ml, calculate the molarity of acetic acid in vinegar...
Questions



Mathematics, 10.01.2021 23:50


Mathematics, 10.01.2021 23:50


English, 10.01.2021 23:50

Mathematics, 10.01.2021 23:50

Social Studies, 10.01.2021 23:50

Mathematics, 10.01.2021 23:50


Arts, 10.01.2021 23:50

Spanish, 10.01.2021 23:50

Mathematics, 10.01.2021 23:50

History, 10.01.2021 23:50


Mathematics, 10.01.2021 23:50



Mathematics, 10.01.2021 23:50

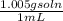 =1005 g soln
=1005 g soln

