Chemistry, 29.07.2019 16:30 angelinacortes11
Consider the unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α, β, γ, δ = request answer part b determine how many moles of o2 are required to react completely with 6.4 moles c6h14. express your answer using two significant figures. n = mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Consider the unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) par...
Questions

Mathematics, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00



English, 09.05.2021 14:00

Biology, 09.05.2021 14:00

Physics, 09.05.2021 14:00

Biology, 09.05.2021 14:00

Spanish, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

English, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00


Mathematics, 09.05.2021 14:00

Computers and Technology, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00

Mathematics, 09.05.2021 14:00


History, 09.05.2021 14:00

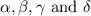 are 2, 19, 12 and 14 respectively.
are 2, 19, 12 and 14 respectively.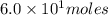 of
of 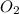 are required to react with 6.4 moles of
are required to react with 6.4 moles of 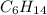 .
.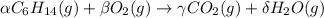
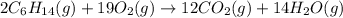
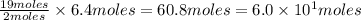 of
of