
Chemistry, 29.07.2019 13:30 aurelio1121
Radon (rn) is the heaviest and the only radioactive member of group 8a(18), the noble gases. it is a product of the disintegration of heavier radioactive nuclei found in minute concentrations in many common rocks used for building and construction. in recent years, health concerns about the cancers caused from inhaled residential radon have grown. if 1.00 × 1015 atoms of radium (ra) produce an average of 1.373 × 104 atoms of rn per second, how many liters of rn, measured at stp, are produced per day by 9.64 g of ra?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Radon (rn) is the heaviest and the only radioactive member of group 8a(18), the noble gases. it is a...
Questions

Mathematics, 18.02.2022 21:00


Physics, 18.02.2022 21:00












Biology, 18.02.2022 21:00



Business, 18.02.2022 21:00

Physics, 18.02.2022 21:00

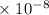 L.
L.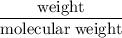
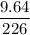
 Avagadro number
Avagadro number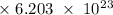
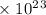
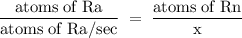
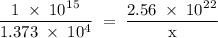
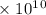 atoms/sec.
atoms/sec.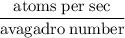
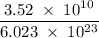 moles
moles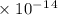 mol/sec.
mol/sec.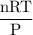
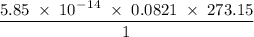
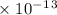 liter/sec.
liter/sec.


