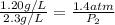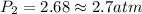Chemistry, 29.07.2019 13:00 ashleyann3052
To increase the solubility of a gas at constant temperature from 1.20 g/l, at 1.4 atm, to 2.3 g/l, the pressure would have to be increased to 2.7 atm 0.7 atm 0.37 atm 1.37 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3


Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3
You know the right answer?
To increase the solubility of a gas at constant temperature from 1.20 g/l, at 1.4 atm, to 2.3 g/l, t...
Questions

Mathematics, 16.04.2021 19:10



Biology, 16.04.2021 19:10




History, 16.04.2021 19:10


Mathematics, 16.04.2021 19:10


Mathematics, 16.04.2021 19:10

Mathematics, 16.04.2021 19:10


Mathematics, 16.04.2021 19:10

English, 16.04.2021 19:10


Mathematics, 16.04.2021 19:10

Mathematics, 16.04.2021 19:10


 = initial solubility of gas = 1.20 g/L
= initial solubility of gas = 1.20 g/L
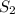 = final solubility of gas = 2.3 g/L
= final solubility of gas = 2.3 g/L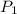 = initial pressure of gas = 1.4 am
= initial pressure of gas = 1.4 am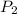 = final pressure of gas = ?
= final pressure of gas = ?