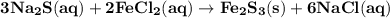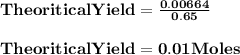Chemistry, 29.07.2019 09:00 josephaciaful
How many milliliters of 0.200 m fecl3 are needed to react with an excess of na2s to produce 1.38 g of fe2s3 if the percent yield for the reaction is 65.0%? 3 na2s(aq) + 2 fecl3(aq) → fe2s3(s) + 6 nacl(aq)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2


Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
How many milliliters of 0.200 m fecl3 are needed to react with an excess of na2s to produce 1.38 g o...
Questions

Mathematics, 11.09.2021 05:00

English, 11.09.2021 05:00

Biology, 11.09.2021 05:00

Mathematics, 11.09.2021 05:00



English, 11.09.2021 05:00

Mathematics, 11.09.2021 05:00

Mathematics, 11.09.2021 05:00

History, 11.09.2021 05:00



Mathematics, 11.09.2021 05:00





Mathematics, 11.09.2021 05:00

Mathematics, 11.09.2021 05:00

Mathematics, 11.09.2021 05:00

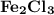 is needed to react with an excess of
is needed to react with an excess of 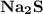 to produce 1.38 g of
to produce 1.38 g of 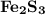 .
.