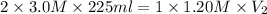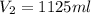Chemistry, 29.07.2019 01:30 kleathers97
Automobile batteries use 3.0 m h2so4 as an electrolyte. how much 1.20 m naoh will be needed to neutralize 225 ml of battery acid? h2so4(aq) + 2naoh(aq) --> 2h2o(l) + na2so4(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
Automobile batteries use 3.0 m h2so4 as an electrolyte. how much 1.20 m naoh will be needed to neutr...
Questions


Mathematics, 22.12.2020 23:00

Mathematics, 22.12.2020 23:00


History, 22.12.2020 23:00

Mathematics, 22.12.2020 23:00

Spanish, 22.12.2020 23:00

Chemistry, 22.12.2020 23:00

English, 22.12.2020 23:00

Mathematics, 22.12.2020 23:00

English, 22.12.2020 23:00


Arts, 22.12.2020 23:00

Mathematics, 22.12.2020 23:00



English, 22.12.2020 23:10

Mathematics, 22.12.2020 23:10


Mathematics, 22.12.2020 23:10

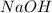 needed are, 1125 ml
needed are, 1125 ml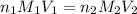
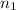 = basicity of an acid = 2
= basicity of an acid = 2
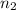 = acidity of a base = 1
= acidity of a base = 1
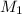 = concentration of
= concentration of 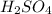 = 3.0 M
= 3.0 M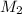 = concentration of NaOH = 1.20 M
= concentration of NaOH = 1.20 M
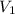 = volume of
= volume of 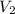 = volume of NaOH = ?
= volume of NaOH = ?