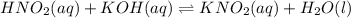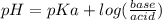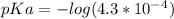Chemistry, 25.10.2019 03:43 vismayagejjala
What is the ph at the half-stoichiometric point for the titration of 0.22 m hno2(aq) with 0.1 m koh(aq)? for hno2, ka = 4.3x10-4?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
What is the ph at the half-stoichiometric point for the titration of 0.22 m hno2(aq) with 0.1 m koh(...
Questions





History, 17.07.2020 20:01




History, 17.07.2020 20:01

Mathematics, 17.07.2020 20:01







History, 17.07.2020 20:01


Mathematics, 17.07.2020 20:01

Mathematics, 17.07.2020 20:01

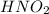 is written as:
is written as: