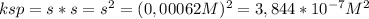(a) at 10°c, 8.9 10−5 g of agcl(s) will dissolve in 100. ml of water. (i) write the equation for the dissociation of agcl(s) in water. (ii) calculate the solubility, in mol l-1, of agcl(s) in water at 10°c. (iii) calculate the value of the solubility-product constant, ksp, for agcl(s) at 10°c. (b) at 25°c, the value of ksp for pbcl2(s) is 1

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Subduction zones occur on earth where dense oceanic crust dives under more buoyant continental crust. these boundaries are characterized by a deep ocean trench next to a high continental mountain range, large numbers of earthquakes and volcanoes. all of this is further evidence for the a) big bang theory b) origin of the species eliminate c theory of plate tectonics d theory of natural selection 4 sedimentary rocks found high in the himalayen mountain
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
(a) at 10°c, 8.9 10−5 g of agcl(s) will dissolve in 100. ml of water. (i) write the equation for the...
Questions


Mathematics, 19.07.2019 03:30



Computers and Technology, 19.07.2019 03:30


History, 19.07.2019 03:30

Social Studies, 19.07.2019 03:30

Mathematics, 19.07.2019 03:30

Mathematics, 19.07.2019 03:30

Computers and Technology, 19.07.2019 03:30

Business, 19.07.2019 03:30

English, 19.07.2019 03:30

Biology, 19.07.2019 03:30

Spanish, 19.07.2019 03:30

Chemistry, 19.07.2019 03:30




History, 19.07.2019 03:30

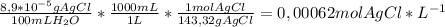
![ksp= [Ag^{+}]*[Cl^{-}]](/tpl/images/0144/2915/6d867.png)