

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
Aluminum is produced commercially by the electrolysis of al2o3 in the presence of a molten salt. if...
Questions

Mathematics, 23.10.2020 05:01



Mathematics, 23.10.2020 05:01

Advanced Placement (AP), 23.10.2020 05:01



History, 23.10.2020 05:01

English, 23.10.2020 05:01

Mathematics, 23.10.2020 05:01

Mathematics, 23.10.2020 05:01

Mathematics, 23.10.2020 05:01


Mathematics, 23.10.2020 05:01




History, 23.10.2020 05:01

Mathematics, 23.10.2020 05:01

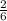 = 3.83 x 10⁴ mole of Al
= 3.83 x 10⁴ mole of Al


