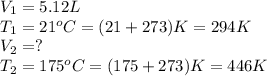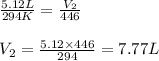Chemistry, 28.07.2019 07:00 bryson9604
Afixed quantity of gas at 21 °c exhibits a pressure of 752 torr and occupies a volume of 5.12 l. (a) calculate the volume the gas will occupy if the pressure is increased to 1.88 atm while the temperature is held constant. (b) calculate the volume the gas will occupy if the temperature is increased to 175 °c while the pressure is held constant.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
Afixed quantity of gas at 21 °c exhibits a pressure of 752 torr and occupies a volume of 5.12 l. (a)...
Questions

Mathematics, 03.03.2021 03:50


Physics, 03.03.2021 03:50


Mathematics, 03.03.2021 03:50

Mathematics, 03.03.2021 03:50


Mathematics, 03.03.2021 03:50


Social Studies, 03.03.2021 03:50


Biology, 03.03.2021 03:50

English, 03.03.2021 03:50

English, 03.03.2021 03:50

History, 03.03.2021 03:50

History, 03.03.2021 03:50


Mathematics, 03.03.2021 03:50


Mathematics, 03.03.2021 03:50

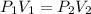
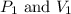 are initial pressure and volume.
are initial pressure and volume.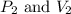 are final pressure and volume.
are final pressure and volume.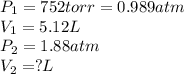
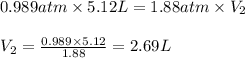
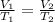
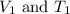 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.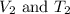 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.