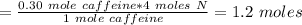Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
How many mol of nitrogen is in 0.30 mol of caffeine...
Questions

Social Studies, 16.09.2020 06:01

English, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Physics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

English, 16.09.2020 06:01

English, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01