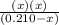Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Find the h3o+ concentration of a 0.210 m hypochlorous acid solution (whose acid dissociation constan...
Questions





Social Studies, 23.05.2020 20:00

Mathematics, 23.05.2020 20:00

Mathematics, 23.05.2020 20:00

English, 23.05.2020 20:00

Mathematics, 23.05.2020 20:00



English, 23.05.2020 20:00

Physics, 23.05.2020 20:00



Mathematics, 23.05.2020 20:00

Mathematics, 23.05.2020 20:00


Mathematics, 23.05.2020 20:00

Social Studies, 23.05.2020 20:00

![\frac{[H_{3}O^+][ClO^-]}{[HClO]}](/tpl/images/0141/2399/f560a.png)