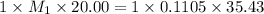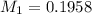You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you will titrate this with a 0.1105 m naoh. you add 20.00 ml of hcooh to the beaker before titrating, and it requires 35.43 ml of naoh to reach the end point. what is the concentration of the hcooh solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


You know the right answer?
You are given a solution of hcooh (formic acid) with an approximate concentration of 0.20 m and you...
Questions



History, 17.07.2019 19:30

History, 17.07.2019 19:30

English, 17.07.2019 19:30

History, 17.07.2019 19:30

Mathematics, 17.07.2019 19:30

English, 17.07.2019 19:30

English, 17.07.2019 19:30


Biology, 17.07.2019 19:30


Mathematics, 17.07.2019 19:30

Biology, 17.07.2019 19:30


History, 17.07.2019 19:30


Mathematics, 17.07.2019 19:30



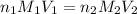
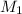 = molarity of
= molarity of 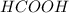 solution = ?
solution = ?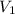 = volume of
= volume of 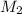 = molarity of
= molarity of 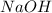 solution = 0.1105 M
solution = 0.1105 M
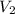 = volume of
= volume of 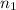 = valency of
= valency of 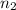 = valency of
= valency of