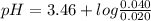Chemistry, 27.07.2019 19:30 brittanyfox411
Calculate the ph of a buffer that is 0.020 m hf and 0.040 m naf. the ka for hf is 3.5 × 10-4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
Calculate the ph of a buffer that is 0.020 m hf and 0.040 m naf. the ka for hf is 3.5 × 10-4....
Questions




Mathematics, 15.10.2020 17:01

English, 15.10.2020 17:01

Mathematics, 15.10.2020 17:01



Social Studies, 15.10.2020 17:01

Mathematics, 15.10.2020 17:01




Computers and Technology, 15.10.2020 17:01



History, 15.10.2020 17:01


English, 15.10.2020 17:01

Physics, 15.10.2020 17:01

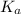 of HF is
of HF is 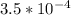
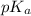 :
: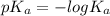
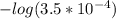
![pH = pK_{a} + log\frac{[Base]}{[Acid]}](/tpl/images/0139/8795/8fa71.png)