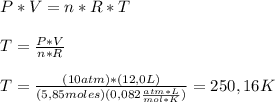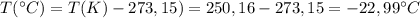Chemistry, 27.07.2019 17:30 takaralocklear
Calculate the temperature of 5.85 mol n2 gas in 12.0 l steel bottle under the 10.0 atm of pressure.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2


Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Calculate the temperature of 5.85 mol n2 gas in 12.0 l steel bottle under the 10.0 atm of pressure....
Questions


Mathematics, 22.02.2021 21:50

English, 22.02.2021 21:50

Social Studies, 22.02.2021 21:50


History, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50

History, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50


Mathematics, 22.02.2021 21:50

Mathematics, 22.02.2021 21:50




Health, 22.02.2021 21:50


Biology, 22.02.2021 21:50


Mathematics, 22.02.2021 21:50