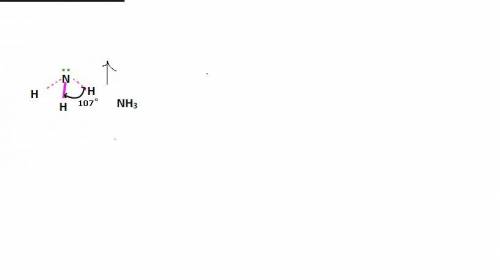
Chemistry, 27.07.2019 17:30 nockturnal1993
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride has a central boron atom bonded to three fluorine atoms. however, ammonia is pyramidal and boron trifluoride is trigonal planar in shape. which statement justifies this difference in their structure? the lone pair of electrons in boron trifluoride increases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride decreases the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in boron trifluoride stabilizes the bond angle between the bonding pairs, giving it a planar structure. the lone pair of electrons in ammonia increases the bond angle between the bonding pairs, giving it a pyramidal structure. the lone pair of electrons on the nitrogen in ammonia decreases the bond angle between the bonding pairs, giving it a pyramidal structure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
In ammonia, a central nitrogen atom is bonded to three hydrogen atoms. similarly, boron trifluoride...
Questions

Mathematics, 17.01.2021 06:30

Geography, 17.01.2021 06:30

Mathematics, 17.01.2021 06:30


Mathematics, 17.01.2021 06:30

Physics, 17.01.2021 06:30

Physics, 17.01.2021 06:40


Physics, 17.01.2021 06:40

Mathematics, 17.01.2021 06:40

Mathematics, 17.01.2021 06:40


Chemistry, 17.01.2021 06:40


Mathematics, 17.01.2021 06:40

Mathematics, 17.01.2021 06:40

Mathematics, 17.01.2021 06:40

Mathematics, 17.01.2021 06:40


Mathematics, 17.01.2021 06:40

![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0139/5742/08b6a.png)
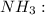
![{\text{Number of electrons}} =\frac{1}{2}[5+3-0+0]=4](/tpl/images/0139/5742/46c6e.png)
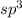 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
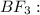
![{\text{Number of electrons}} =\frac{1}{2}[3+3-0+0]=3](/tpl/images/0139/5742/beb45.png)
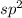 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.