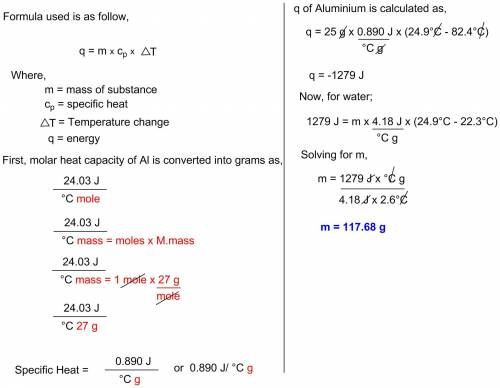
A12.8 g piece of aluminum (which has a molar heat capacity of 24.03 j/°c·mol) is heated to 82.4°c and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/g°c) initially at 22.3°c. the final temperature of the water is 25.7°c. ignoring significant figures, calculate the mass of water in the calorimeter.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
A12.8 g piece of aluminum (which has a molar heat capacity of 24.03 j/°c·mol) is heated to 82.4°c an...
Questions


Social Studies, 10.09.2021 04:50


English, 10.09.2021 04:50


History, 10.09.2021 04:50

English, 10.09.2021 04:50



Mathematics, 10.09.2021 04:50




Physics, 10.09.2021 04:50

Mathematics, 10.09.2021 04:50

Health, 10.09.2021 04:50

English, 10.09.2021 04:50


English, 10.09.2021 04:50

Mathematics, 10.09.2021 04:50